| 1. 基本定義: |
| |
| A.
相律 (相、成分與自由度) |
 系統
(system) 系統
(system) |
定義—
Any portion of the material universe which can be isolated completely
and arbitrarily from
the rest for consideration of the changes which may occur within it
under varying conditions. |
例如—the
binary system CaO-Al2O3
the
ternary system K2O-B2O3-H2O |
 相
(P, phase) 相
(P, phase) |
定義—
Any portion of a system which is physically homogeneous within itself
and bounded by a
surface so that it is mechanically separable from any other portions. |
| Homogeneous:
系統僅由一相組成。 |
| Heterogeneous:
系統由多相組成。 |
 成分
(C, composition) 成分
(C, composition) |
定義—
The smallest number of independently variable chemical constituents necessary
and sufficient
to express the composition of each phase present in any state of equilibrium. |
| (在表示一個相的化學組成時,允許使用零或負數的成分。) |
 自由度
(F, degree of freedom, or variance) 自由度
(F, degree of freedom, or variance) |
定義—
The number of intensive variables which can be altered inde-pendently and
arbitrarily without
bring about the disappearance of a phase or the formation of a new one. |
|
intensive variable : 與質量和系統大小無關的變量。例如:
P,T,化學組成等。 |
|
invariant : F = 0 |
|
mono-variant : F = 1 |
|
bi-variant :
F = 2 |
|
tri-variant : F = 3 |
 相律
(phase rule) 相律
(phase rule) |
| P
+ F = C + 2 (口訣:police force = cops + 2) |
| 當系統是一個condensed
system時,相律會成為: |
| P
+ F = C + 1 |
| |
| B. 相律的限制 |
| 只適用於一系統的平衡狀態。 |
| 只與成分和相的數目有關。 |
| 與各成分的本質無關,與各相的數量和本質也無關。 |
| 不能告訴我們反應的速率。 |
| |
| C.
與微構造的關係 |
| 5. 相圖的製作 |
| |
| A.
計算 – 簡單系統。 |
| B.
實驗 |
| 三種判斷反應是否達到平衡的方法:
|
| (1)
時間 (2) 雙向反應 (3) 不同實驗步驟 |
| C.
容易犯的錯誤 |
| 四種基本規則: |
| (1)
相律 (The Phase Rule) |
| (2)
邊界規則 (The Boundary Rule) |
Any
p-phase region can be bounded only by regions containing p 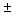 1
phases 1
phases |
| (3)
邊界曲線規則 (The Boundary-curvature Rule) |
•
Boundaries of one-phase regions must meet with curvatures such that the
boundaries extrapolate
into
the adjacent two-phase regions. |
•
The included angle within a one-phase region between two boundaries of the
region must be
less than
180o at the intersection of the boundaries. |
| (4)
溶解度規則 (The Solubility Rule) |
| All
components are soluble to some degree in all phases. |